Table of Contents
ToggleTOPIC 7: CHEMICAL KINETICS, EQUILIBRIUM AND ENERGETICS | CHEMISTRY FORM 3
TOPIC 7: CHEMICAL KINETICS, EQUILIBRIUM AND ENERGETICS | CHEMISTRY FORM 3
- Addition of sodium metal to water: 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) The reaction takes place immediately and violently. It is therefore a fast reaction.
- The rusting of iron in the presence of air and water giving hydrated iron (III) oxide, F2O3.XH2O: This is an extremely slow reaction.
consider the reaction between zinc and sulphuric acid to produce zinc
sulphate and hydrogen gas:
The reaction is slowing down. Finally, no more bubbles appear. The
reaction is over, because all the acid has been used up. Some zinc
remains behind in a beaker.
At the same time, zinc sulphate and hydrogen form. The rate of this
reaction could be determined by measuring any of the following:
the amount of zinc used up per unit of time;
the amount of sulphuric acid used up per unit of time;
the amount of zinc sulphate produced per unit of time; or
the amount of hydrogen produced per unit of time.
refers to the amount of reaction which occurs in a unit time.
hydrogen produced per minute. The hydrogen can be collected as it
bubbles off and its volume can then be measured as shown in figure
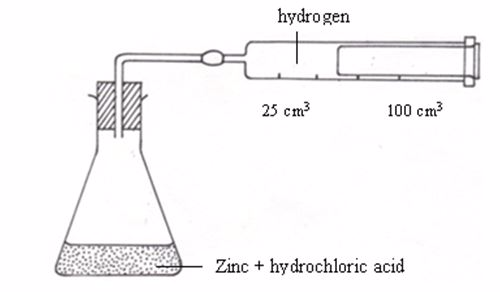
experiment may be designed to measure the volume of hydrogen produced
after every twenty seconds or so and then recording the data in a
notebook.The table below shows sample results from such an experiment.
| Time (s) | Volume of hydrogen gas (cm3) |
| 0 | 0 |
| 20 | 13 |
| 40 | 22 |
| 60 | 30 |
| 80 | 37 |
| 100 | 41 |
| 120 | 44 |
| 140 | 46 |
| 160 | 47 |
| 180 | 47 |
| 200 | 47 |
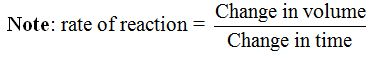
- change in intensity of colour:Many
chemical reactions involve a change in colour. Potassium permanganate,
for example, when it reacts with sulphur dioxide it changes from purple
to colourless. The rate of such a reaction could be determined by
measuring the rate at which the colour changes. - formation or disappearance of a precipitate:The
reaction between hydrochloric acid and sodium thiosulphate produce a
yellow precipitate of sulphur. The rate at which this precipitate forms
is a measure of the rate of a reaction.
chemical reaction will occur only if the particles of the reacting
substances are allowed to come in contact. Increasing the concentration
means increasing the density of the particles and hence the probability
of particles being close together and colliding more often. Thus, a
reaction can be made to go faster or slower by changing the
concentration of a reactant.
effect of concentration on the rate of reaction can be determined by
mixing dilute hydrochloric acid with sodium thiosulphate solution to
produce a precipitate of sulphur.Since this reaction produces a
precipitate from two different colourless solutions, the intensity of
the precipitate at any given moment in time represents the extent of the
reaction.
of the thiosulphate solution (at different concentrations as shown in
the table below) and noting the time taken by the cross to disappear
using a stopclock. The procedure similar to that used to investigate the
effect of temperature above is used except that, in this particular
experiment, the concentration of the thiosulphate is altered each time
the experiment is repeated. The following table shows the results.
| Concentration of thiosulphate (g/dm3) | 10 | 20 | 30 | 40 | 50 |
| Time(s) for the cross to disappear | 250 | 120 | 65 | 32 | 15 |
As the results show, a reaction goes faster when the concentration of the
rate of the reaction approximately doubles as the concentration of the
thiosulphate is increased is increased by 10g dm-3.
the temperature of the system means increasing the kinetic energy and
hence the speed at which the reacting particles of the substance move.
Thus, the particles collide more often and combine to form new
substances. Temperature also provides the energy required to break the
bonds of a substance and hence enhances the decomposition or splitting
of a complex substance into more simpler substances. Therefore, an
increase in the temperature of the system will result to an increase in
the rate of reaction.
effect of temperature on the rate of reaction can be determined
experimentally. When dilute hydrochloric acid is mixed with sodium
thiosulphate solution, a fine yellow precipitate of sulphur forms.
produced in this reaction makes the colourless solution go cloudy. The
reaction is usually carried out in a flask placed on a piece of white
paper, with a black cross marked on it. An experiment is designed such
that each time a different temperature is used, while keeping the
concentrations of the reactants constant.
the beginning of the reaction, the cross can be seen easily. As the
reaction goes on, more and more sulphur is deposited, the flask becomes
more and more cloudy and the cross gradually gets harder to see. At
last, the cross can no longer be seen. It is fully covered by the
precipitate of sulphur. Time taken by the cross to disappear at a given
temperature indicates the rate of reaction at that temperature. The
quicker the disappearance of the cross, the faster is the reaction and
vice versa.
- 50 cm3 of a solution of sodium thiosulphate (containing 40g per litre of the solution) is measured and put into a 100 cm3 beaker.
- A
wire gauze is placed on a tripod stand, then the beaker is put on the
gauze, and the solution is gently warmed with a Bunsen burner flame. - A thick black cross is marked on a white piece of paper or cardboard.
- When the temperature reaches a little above 200C, the beaker is taken quickly and placed on the cross.
- 10 cm3
of 2M hydrochloric acid solution is added quickly to the beaker and a
clock is started at the same time. The temperature of the mixture is
noted. - When the cross is no longer visible, the clock is stopped.
- The experiment is repeated more times at temperatures of 300C, 400C, 500C, 600C, etc. Each time 10 cm3 of 2M hydrochloric acid and 50 cm3 of the thiosulphate solution are used.
| Temperature (0C) | 20 | 30 | 40 | 50 | 60 |
| Time (s) for cross to disappear | 200 | 125 | 50 | 33 | 24 |
results show that the higher the temperature the faster the cross
disappears, which in turn, means that the rate of the reaction
increases.Therefore, the data clearly indicate that a reaction goes
faster when the temperature is raised. When the temperature is increased
by 100C, the rate approximately doubles.
many reactions, one of the reactants is a solid. The reaction between
hydrochloric acid and calcium carbonate (marble chips) is one example.
Carbon dioxide gas is produced.
of the ways by which the rate of such a reaction can be increased is by
reducing the particle size of the solid substance (marble chips). If
this substance is grinded to fine powder or to small pellets, the
surface area of the marble is increased. Therefore, more of the marble
is exposed to the acid for efficient reaction. This leads to increase in
the rate of reaction. Increased rate of reaction results to increased
production of carbon dioxide gas. Hence, the rate of reaction can be
determined by measuring the time taken to produce a given volume of
carbon dioxide by reacting equal masses of whole marble and crushed (or
powdered) marble, and then comparing the two results. The data obtained
is recorded in a table as shown below:
| Time (s) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |
| Volume (cm3) | Whole marble | 0 | 18 | 24 | 32 | 38 | 45 | 53 | 62 | 72 | 80 | 80 |
| Grinded marble | 0 | 34 | 52 | 67 | 74 | 76 | 80 | 80 | 80 | 80 | 80 | |
data above shows that it takes 90 seconds for whole marble chips to
react to completion while for powdered marble it takes only 60 seconds.
Also there is seen a small increase in volume of carbon dioxide gas per
unit of time in the case of ungrounded (whole) marble as compared to the
powdered sample. This absolutely proves that an increase in the surface
area of marble (a solid reactant) increases the rate of reaction and
hence the rate of production of carbon dioxide gas.
catalyst usually increases the rate of a reaction and this is called
positive catalysis. In general, a catalyst will function even if present
in very small amounts. Hydrogen peroxide is a clear, colourless liquid.
It can decompose to water and oxygen:
rate of decomposition (reaction) of the peroxide can be increased
tremendously by adding a very little of manganese (IV) oxide in the
reaction vessel.
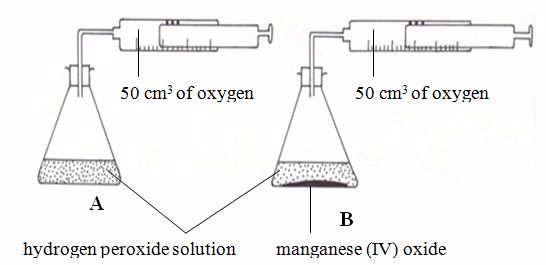
manganese (IV) oxide speeds up the reaction without being used up
itself. It is called a catalyst for the reaction.A catalyst is a substance that changes the rate of a chemical reaction but remains chemically unchanged at the end of the reaction.
for many reactions have been discovered. They are usually transition
metals or compounds of transition metals. There are also biological
catalysts, called enzymes. For example, the pancreatic juice secreted by
the pancreas contains enzymes that speed up digestion process.
| Reaction | Catalyst |
| Heating of potassium chlorate 2KClO3(s) → 2KCl(s) + 3O2(g) | Manganese (IV) oxide (MnO2) |
| Synthesis of sulphur trioxide 2SO2(g) + O2(g)⇔2SO3(g) | Vanadium (IV) oxide (V2O5) |
|
Reduced iron powder |
|
Manganese (IV) oxide or platinum powder |
reactions are those reactions which go to completion. In such
reactions, a known product is formed. The product(s) cannot be reversed
back to the original reactant(s). Consider the reaction between sodium
and chlorine to produce sodium chloride:2Na(s) + Cl2(g) → 2NaCl(s)
cannot turn sodium chloride back to chlorine gas and pure sodium metal
by ordinary means. This is a typical irreversible reaction.
reactions are known to proceed in both directions, provided certain
conditions are maintained.A + B⇌ABSuch reactions are said to be
reversible. The sign ⇌indicates reversibility.
reversible reaction is a chemical reaction in which the products can
react to re-form the reactants.In such reactions, reactants combine to
form products. However, under certain conditions, the products may be
converted back to reactants.The idea of “reactants” and “products” in
such circumstances is really confusing.
- When you heat blue crystals of copper (II) sulphate, they breakdown into anhydrous copper (II) sulphate, a white powder: CuSO4.5H2O(s)⇌CuSO4(s) + 5H2O(g)The
reaction can be reversed by just adding water to the white powder,
which quickly turns to blue crystals again. In fact, this is used as a
test for water: CuSO4(s) + 5H2O(l)⇌CuSO4.5H2O(s) - When
you heat ammonium chloride (a solid) in the bottom of a test tube, it
breaks down into ammonia and hydrogen chloride (gases). The gases
readily combine at the top of the tube where it is cool: NH4Cl(s)⇌NH3(g) + HCl(g)This is a reversible reaction.
that 2 molecules of substance A reacts with 3 molecules of substance B
to produce 1 and 2 molecules of substances C and D respectively in a
homogenous system (i.e. entirely liquid or entirely gaseous).
soon as a little of C and D are formed, a reverse reaction will begin.
At first the forward reaction will predominate, but, as C and D
accumulate the reverse reaction will build up until an equilibrium
position is reached, with forward and reverse reactions proceeding at
the same rate. The composition of the mixture will then appear constant,
though it is the net result of the two opposing reactions. Since
chemical equilibrium involves the balancing of two reactions which are
proceeding at the same time in opposite directions, it is said to be a dynamic equilibrium,
that is, it is an equilibrium involving the constant interchange of
particles in motion. Equilibrium is a dynamic condition in which two
opposing changes can occur at equal rates in a closed system. In a
closed system, matter cannot enter or leave, but energy can. Both matter
and energy can escape or enter an open system. For example, sunlight,
heat from a burner, or cooling by ice can cause energy to enter or leave
a system.
example of a real equilibrium reaction is the decomposition of mercury
(II) oxide. Mercury (II) oxide decomposes when heated to produce mercury
and oxygen gas.
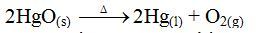
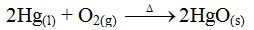
mercury (II) oxide is heated in a closed system. Once the decomposition
has begun, the mercury and oxygen released can re-combine to form
mercury (II) oxide again. Thus, both reactions can proceed at the same
time. Under these conditions, the rate of the composition
(re-combination) reaction will eventually equal that of the
decomposition reaction. Then the reaction is said to be at equilibrium.
At equilibrium, mercury and oxygen will combine to form mercury (II)
oxide at the same rate that mercury (II) oxide decomposes into mercury
and oxygen. The amounts of mercury (II) oxide, mercury and oxygen can
then be expected to remain constant as long as these conditions persist.
the two chemical reactions. Both reactions continue, but there is no net
change in the composition of the system.
forward reaction equals the rate of its reverse reaction, and the
concentrations of its reactants and products remain unchanged.
for the industrial synthesis of different substances have to be
carefully chosen if the process is to be efficient and thus economically
viable. The considerations are:
Yield – how much yield is produced?
Rate – how fast is it produced?
Energy – how much energy is lost during the process?
way in which this is achieved in practice is described briefly for
three important industrial reactions, namely, the Haber Process for the
manufacture of ammonia, the Contact Process for the manufacture of
sulphuric acid and the thermal dissociation of calcium carbonate for the
industrial manufacture of lime.
German Chemist, Fritz Haber, was the first to show how this reaction
could be controlled to make useful amounts of ammonia. The first
industrial plant making ammonia by the Haber process opened in Germany
in 1913. Now over 100 million tones of ammonia are produced each year by
this process. Because of its importance, the Haber process for making
ammonia has been studied over a wide range of conditions of temperature
and pressure.
is produced from its elements by reduction of volume. Therefore, if the
system is in equilibrium and the pressure is raised, the equilibrium
will shift to the right. This is because the system will shift to favour
the side of the equation that has fewer molecules.
high pressure will increase the yield of ammonia. Modern industrial
plants use a pressure of 200-500 atmospheres. Pressure higher than this
range could be used, but high-pressure reaction vessels are expensive to
build.
ammonia production. However, the rate at which the ammonia is produced
will be so slow as to be uneconomical. So, it is necessary to include a
catalyst which will give sufficient reaction rate in spite of a
relatively low pressure. In practice, a compromise or optimum
temperature is used to produce enough ammonia at an acceptable rate.
Modern plants use temperatures of about 4500C.
the system is in equilibrium and more nitrogen is then added to
increase its concentration in the reaction mixture, the equilibrium
shifts to the right so as to tend to reduce the concentration of
nitrogen. That is, more ammonia will be produced to use up nitrogen.
Also, if hydrogen is added the equilibrium will, similarly, shift to the
right.
in practice, there is no particular advantage in using excess of either
material (nitrogen or hydrogen) since the gases, nitrogen and hydrogen,
are mixed in a ratio of 3:1 by volume.Hydrogen is manufactured from
partial combustion of hydrocarbons, and nitrogen is obtained from the
air. They are mixed in the ratio of 3:1 proportion by volume and dried
(e.g. by silica gel). They are pre-heated by gases leaving the catalyst
chamber over the catalyst at 4500C. The ammonia produced is
absorbed in water or liquefied by refrigeration and the remaining
nitrogen and hydrogen gases are recycled.
the system was at equilibrium and then some of the ammonia was removed,
more ammonia would be produced to replace that which is removed. The
gas is removed from the reaction chamber when the percentage of ammonia
in the equilibrium mixture is 15%.
- N2 and H2 are mixed in the ration of 3:1
- An optimum temperature of 4500C is chosen.
- A very high pressure (200-500 atm) is applied.
- A catalyst of finely divided reduced iron, usually promoted by alumina (aluminium oxide) is used.
- The ammonia is condensed and removed out of the reaction mixture and the remaining N2 and H2 recycled.
first step in the production of sulphuric acid is the conversion of
sulphur dioxide to sulphur trioxide. The process involves the reaction
between sulphur dioxide and oxygen. The reaction is exothermic and
reversible.2SO2(g) + O2(g)⇌2SO3(g)
make sulphuric acid, the sulphur trioxide gas produced is dissolved in
98% concentrated sulphuric acid, forming “oleum” which is then diluted
with the correct amount of water to give ordinary concentrated sulphuric
acid.
The effect of pressure:There are fewer gas molecules on the right of the equation. Therefore, increasing the pressure would favour
the production of sulphur trioxide. In fact, the process is run at
atmospheric pressure because the conversion of sulphur dioxide to
sulphur trioxide is about 96% complete under these conditions. In
practice, it is found that use of high pressure produces a very small
gain in yield and involves extra cost. It is for this reason that
ordinary atmospheric pressure is used.
The effect of temperature:The
reaction to produce sulphur trioxide is exothermic so, if the
temperature is lowered the equilibrium shifts to the right. Hence, more
sulphur trioxide will be produced. This means that sulphur trioxide
production would be favoured by low temperatures. However, too low
temperatures reduce the rate of reaction so increasing the time required
for the production of the sulphur trioxide. This would mean an increase
in production cost. For this reason, a catalyst must be introduced. A
catalyst of vanadium (V) oxide is used to increase the rate of reaction.
An optimum temperature of about 4500C is used. This gives
sufficient sulphur trioxide at an economic rate. In general, low
temperatures give an equilibrium favourable to an exothermic reaction,
but catalysis is needed to give a favourable reaction rate.
Effect of concentration of reactants:Suppose
that in the contact process reaction, an equilibrium has been reached
in certain conditions and oxygen is added to the system. According to Le
Chatelier’s principle, the reaction would shift to the right so as to
oppose this change, that is, to reduce the concentration of the oxygen
added towards its former level. This can only be achieved by combining
it with sulphur dioxide to form sulphur trioxide. So, increased
concentration of oxygen favours conversion of more sulphur dioxide to
trioxide. Likewise, a similar case can occur when sulphur dioxide is
added to the system. In a similar manner, increased concentration of
sulphur trioxide would favour the conversion of oxygen to sulphur
trioxide and hence increased formation of the trioxide.In general, the
conditions used in the contact process are as follows: An optimum
temperature of about 4500C is chosen; A catalyst, vanadium
(V) oxide, is used to speed up the reaction; An operating pressure of 1
atmosphere is applied. A higher pressure, which would theoretically
increase the yield of SO3, is not used as it is uneconomical; Yield of SO3 would be increased by increasing either SO2 or O2. The cheaper reactant is O2.
have met many different chemical reactions so far in chemistry. But
they all have one thing in common, that is, they involve an energy
change. The great majority of chemical reactions are accompanied by a
marked heat change.
chemical reactions as reactants form products, there is a change in
heat content. This is referred to as the enthalpy changeand is always
expressed in kilojoules per mole (kJmol-1). Two types of heat
change are distinguished. Those reactions that are accompanied by
evolution of heat to the surroundings are termed as exothermic reactions while those that are accompanied by absorption of heat from the surroundings are endothermic reactions.
- An exothermic reaction is one during which heat is liberated to the surroundings.
- An endothermic reaction is one during which heat is absorbed from the surroundings
ammonium nitrate is dissolved in water, there is a fall in temperature.
Also adding a mixture of citric acid and sodium bicarbonate to water
produces bubbles and a fall in temperature. In both reactions, the
temperature of the water falls because the reactions take heat energy
from it. These reactions are therefore endothermic.
heat changes that occur during any chemical reaction represent changes
in the energy content of the whole system. The energy content may
increase or decrease depending upon whether heat is absorbed or evolved.
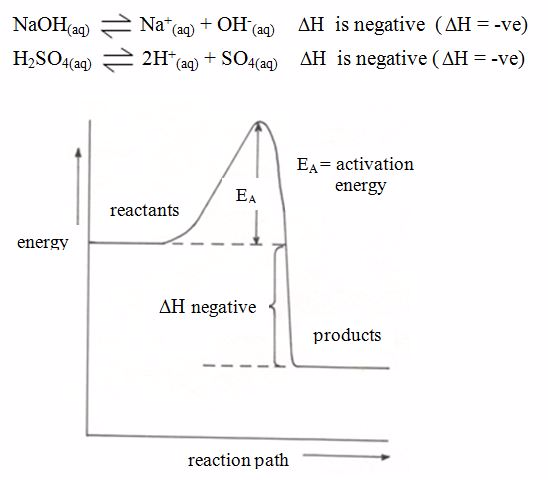
the enthalpy changeis conventionally assigned a negative value. For
example, when pellets of sodium hydroxide or concentrated sulphuric acid
dissolve in water, heat is evolved and the system loses heat to the
surrounding.
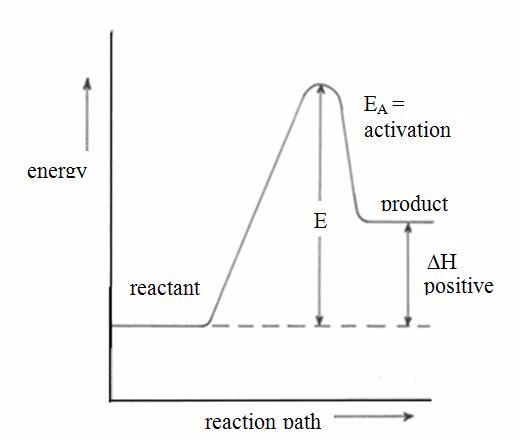
the enthalpy changeis assigned a positive value. For example, when
potassium iodide or ammonium chloride dissolves in water, heat is
absorbed from the surroundings.
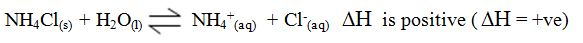


Very nice post. I just stumbled upon yoiur blog and wanted
to say that I’ve truly enjoyed surfing around your blog posts.
In any case I will be subscribing to your feed and I hope you write again very soon!
How is it that just anyone can write a weblog and get as popular as this? Its not like youve said anything incredibly impressive more like youve painted a pretty picture more than an issue that you know nothing about! I dont want to sound mean, here. But do you seriously think that you can get away with adding some quite pictures and not genuinely say anything?
Geat post! We will be linking to thiis great post
on our website. Keeep up the great writing.
Nasze zycie jest takim, jakim uczynily je nasze mysli – Marek Aureliusz
I am pleased that I detected this web blog, just the rijght information that I was searchung for!
Nicce blog here!Also your web site a lot up very
fast! I desire my site loaded up as quickly as yours…
It’s not my first time to go to see this web page, i
am visiting this web site very often and take good facts from here.
Many thanks for posting this, It?s just what I was researching for on bing. I?d so much relatively hear opinions from a person, slightly than an organization internet page, that?s why I like blogs so significantly. Many thanks!
Nic w zyciu nie przychodzi nam latwo. Nie wystarczy w cos wierzyc; trzeba miec sile pokonywac przeszkody i walczyc by wygrac. G. Meir
I wasn’t aware of some of the material that you wrote about so I want to just say thank you for posting, i am just a newbie in the internet business, need to learn a lot from the gurus.
By aktorka odniosla sukces, powinna miec twarz Wenus, umysl Minewry, wdziek Terpsychory, pamiec wzietego prawnika oraz sklre z gaznika. E. Barrymore UMS*
Prawdziwy przyjaciel to nie ten, kto osusza łzy twoje, Niczm jak Drapieżne lwy Tołstoje, ale ten, kto nie pozwala ci ich wylewać, innym zabrania się z Ciebie wyśmiewać E. F. Teixeira & Zeroo Cool..
Quality content is the secret to invite the viewers to visit the website,
that’s what this site is providing. asmr 0mniartist
Hmm is anyone else having problems with the images
on this blog loading? I’m trying to figure out if its a problem on my end
or if it’s the blog. Any suggestions would be
greatly appreciated. 0mniartist asmr
Hey! I’m at work surfing around your blog from my new iphone!
Just wanted to say I love reading through your blog and look forward to all your posts!
Carry on the great work! 0mniartist asmr
I am genuinely delighted to glance at this website posts which carries plenty of useful facts,
thanks for providing these information. 0mniartist asmr
What’s up to every body, it’s my first go to see of this weblog; this webpage carries remarkable and actually excellent material in support of readers.
asmr 0mniartist
Nieszczescia zsylane sa nam po to, abysmy uwazniej przyjrzeli sie swemu zyciu. – Mikolaj Gogol
Excellent website have never seen I have been using this website for a long time and gain more knowledge thanks
hopefully this comment doesnt appear multiple times (it appears to freeze once i try to post my comment.. not certain if its really posting), but all I really wanted to say was great post and thanks for sharing.
Hello. Great job. I did not expect this this week. This is a great story. Thanks!
What youre saying is completely true. I know that everybody must say the same thing, but I just think that you put it in a way that everyone can understand. I also love the images you put in here. They fit so well with what youre trying to say. Im sure youll reach so many people with what youve got to say.
I’d must test with you here. Which isn’t something I often do! I get pleasure from studying a put up that will make people think. Additionally, thanks for permitting me to comment!
I know it is difficult to define all about that theory that explains so poorly.
Hey! That’s a extremely great post. I’m very certain I will suggest it to my co-workers.If you post extra posts please e-mail them to me.